Surely, their versatility is one of the best things about human beings, but when push comes to shove, there is still an awful little that they do better than improving on a consistent basis. This …
Surely, their versatility is one of the best things about human beings, but when push comes to shove, there is still an awful little that they do better than improving on a consistent basis. This ability, in particular, has brought the world some huge milestones, with technology emerging as quite a major member of the group. The reason why we hold technology in such a high regard is, by and large, predicated upon its skill-set, which guided us towards a reality that nobody could have ever imagined otherwise. Nevertheless, if we look beyond the surface for one hot second, it will become abundantly clear how the whole runner was also very much inspired from the way we applied those skills across a real world environment. The latter component, in fact, did a lot to give the creation a spectrum-wide presence, and as a result, initiated a full-blown tech revolution. Of course, the next thing this revolution did was to scale up the human experience through some outright unique avenues, but even after achieving a feat so notable, technology will somehow continue to bring forth the right goods. The same has turned more and more evident in recent times, and assuming one new discovery ends up with the desired impact, it will only put that trend on a higher pedestal moving forward.
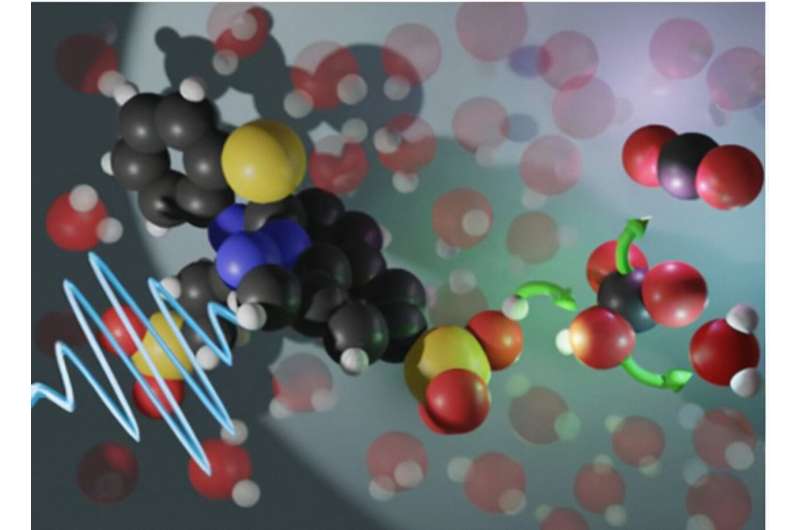
The researching team at Department of Energy’s Oak Ridge National Laboratory has successfully conceived a brand new way to release carbon dioxide (CO2) from a solvent used in direct air capture (DAC) and trap the greenhouse gas for good. Before we get into the discovery itself, we must talk about all those existing methods that we use to perform the stated extraction, You see, the current methods filter it through an aqueous solution containing a sorbent material, such as an amino acid, that takes up atmospheric CO2 and holds it. Next up, heat is roped in to release the carbon dioxide and regenerate the amino acid for recycling purposes. As far as CO2 is concerned, it is either stored or converted into value-added products, such as ethanol, polymers or concrete. Now, as comprehensive and ingenious as they sound, these current methods come with a major energy-related drawback. To offer more details, the process for releasing CO2 and regenerating the sorbent material actually shakes out to be a pretty energy-intensive affair, thus causing all sorts of sustainability concerns. But how does our new technology solve such a massive conundrum? Well, it bets on a novel acid called photoacid, which becomes more acidic when it absorbs light. So, when you expose the acid to something like ultraviolet light, a chemical group in the middle of the acid rotates from the opposite side of a bond to the same side. Forming a ring, the reaction instigates transfer of a proton, or hydrogen ion, to the water solvent. Once the transfer has been duly completed, it bolsters the solution’s acidity levels to produce a change called pH Swing, leaving excess protons to interact with bicarbonate, or HCO3, which was made when CO2 reacted with the sorbent. Following their interaction, the bicarbonate is to accept proton to become carbonic acid, and in other words, a material just one energetically favorable step away from carbon dioxide and water.
In the existing direct-air-capture technologies, CO2 release and sorbent regeneration are the most energy-intensive steps,” said Yingzhong Ma, a chemist at Oak Ridge National Laboratory who led the study. “The goal here is to use the amino acid sorbent, which is recyclable and has a lot of attractive properties, combined with a more energy-efficient approach to release the CO2 and regenerate the sorbent.”
Another major member of the researching team behind this development was a chemist named Radu Custelcean, who led a separate study, back in 2017, where it was found that a guanidine sorbent could directly capture CO2 from air. One year later, he and his team demonstrated a practical and energy-efficient DAC method using solar heat to drive the release of the greenhouse gas from an amino-acid sorbent. After gaining enough confidence regarding its viability, the team would license the technology to a Knoxville-based startup Holocene to prepare it for industrial deployment.
Coming back to the discovery in focus, the researchers faced a huge roadblock during their journey when they tried using conventional photoacids. This was mainly because the lifespan of their excited state was eventually deemed to be too short.
“When carbonic acid decomposes, it has a short lifetime in water, on the order of a few seconds. But that’s an infinity compared to the lifetime of a regular photoacid, which is nanoseconds, or billionths of seconds,” Custelcean said. “That’s why you cannot do this chemistry with a regular photoacid: It takes seconds to release CO2 from carbonic acid, but it takes only nanoseconds for the photoacid to take the proton back.”
The team, however, was able to find an answer in a different class of photoacids called metastable-state photoacid. Boasting a structure that persists in solution from seconds to hours, the stated element ensured the pH change driven by the photoacid’s structural change also lasts a lot longer.
Now, while they did get their breakthrough, the technology will likely need a lot of fine-turning, if it is to reach its optimal version. For instance, the researchers hope to improve the solubility of compounds in water, as well as scale-up the absorption of light from the visible spectrum. Furthermore, they also have a plan to decrease the time required to regenerate the photoacid, and widen our understanding of its long-term stability.