The human life is rooted in a host of different principles, and yet the one in which it is rooted the most is that principle of improving at a consistent pace. We say this because …
The human life is rooted in a host of different principles, and yet the one in which it is rooted the most is that principle of improving at a consistent pace. We say this because the stated principle has already fetched the world some huge milestones, with technology appearing as a rather unique member of the group. The reason why technology’s credentials are so anomalous is purposed around its skill-set, which was unprecedented enough to realize all the possibilities for us that we couldn’t have imagined otherwise. Nevertheless, a closer look should be able to reveal how the whole runner was also very much inspired by the way we applied those skills across a real world environment. The latter component was, in fact, what gave the creation a spectrum-wide presence and made it the ultimate centerpiece of every horizon. Now, having such a powerful tool run the show did expand our experience in many different directions, but even after reaching so far ahead, this prodigious concept called technology will somehow keep on delivering the right goods. The same has grown to become a lot more evident in recent times, and assuming one new discovery pans out just like we envision, it will only propel that trend towards greater heights over the near future and beyond.
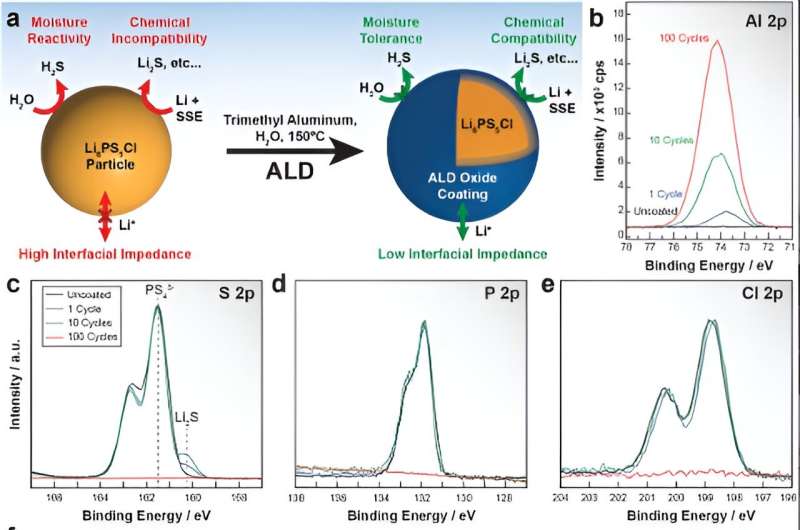
The researching team at Department of Energy’s (DOE) Argonne National Laboratory has successfully conducted an experiment where they took a coating technique long used in manufacturing of computer chips and integrated it into solid-state batteries, doing so to extend the latter’s life. Talk about solid-state batteries for a second, they would become a preferred option over traditional lithium-ion batteries with liquid electrolytes because of all the key advantages they had on the offer. In case you didn’t know, solid-state batteries actually offer us an enhanced brand of safety, an ability to store more energy per unit volume, and the facility to charge more times over their lifetimes. Such capabilities, like you can guess, make them a better alternative for electric cars that are turning increasingly prevalent with time. But how are they able to deliver these advantages? Well, although not entirely, the answer is somewhat rooted in argyrodites, a class of solid-state electrolytes that contain sulfur. You see, argyrodites leverage their higher ionic conductivity to ensure ions can pass through a battery more quickly, thus allowing a faster charge rate. Another detail worth a mention is how argyrodites are much cheaper to process into pellets, a form in which they are ultimately used for manufacturing batteries. However, alongside all these features, argyrodites also present some big manufacturing challenges. Considering they happen to highly reactive with air, it becomes difficult to handle them in a battery production plant. Furthermore, argyrodites react easily with electrode materials such as lithium metal, thus birthing various reaction-induced chemicals that degrade the quality of the electrolyte/electrode interfaces. Not just that, the reaction to emerge here can also slow the movement of lithium ions, diminish battery performance, and form dendrites.
In their response to the challenge in question, the researchers interestingly made a bet on atomic layer deposition, more common within the chip production industry. This coating method basically uses the utility of chemical vapors that react with the surface of a solid material to form a thin film.
“A solid electrolyte’s surface plays a crucial role in how electrolytes and electrodes interact in a battery,” said Justin Connell, an Argonne materials scientist leading the project. “This method allows us to design the surface structure at the atomic level. We believe this fine level of control is needed to optimize the performance of solid-state batteries.”
In practice, the researching team got atomic layer deposition to coat the argyrodite electrolyte in powder form, spelling a significant shift from previous experiments where the coating was done only after the powder form is processed into pellets.
“Coating the pellets would be difficult to scale because they are brittle,” said Connell. “Also, the pellets would need to be coated in batches, and that would increase manufacturing costs.”
They heated the powder and exposed it to water vapor and trimethyl aluminum, producing a thin coating of alumina (aluminum oxide) on all of the individual electrolyte particles. Next up, the team would use a characterization technique called X-ray absorption spectroscopy to determine that the coating did not disrupt the chemical structure of the underlying argyrodite. Then, at Argonne’s Center for Nanoscale Materials, the researchers then used two different techniques to determine how well the coatings conformed to the contours of individual electrolyte particles. During the first technique, named scanning transmission electron microscopy, they created images of the material structure using a focused electron beam. In the second technique, which is called energy-dispersive X-ray spectroscopy, the researchers evaluated the elements in the material by detecting X-rays emitted from electrons used in the earlier scanning transmission electron microscopy method. Finally, the team packaged coated powders into pellets, and incorporated the pellets into a laboratory-scale battery cell with an anode (negative electrode) made of lithium metal.
For the purpose of testing their approach’s success, the researchers repeatedly charged and discharged this battery, along with another battery made with uncoated electrolytes. Taking the available details into account, their discovery showed that the coating significantly decreased the electrolyte’s reactivity with the lithium anode, while simultaneously reducing the rate at which electrons leak out of the electrolyte. The same tests also revealed an unanticipated benefit i.e. the ionic conductivity of the electrolyte was literally doubled by this new method.
When asked to explain the said phenomenon, Peter Zapol, an Argonne physicist and one of the study’s authors, responded as follows:
“We think that the coating is redistributing lithium ions on the electrolyte’s surface and creating more empty spaces along the surface for ions to pass through. These factors may help explain the improved conductivity.”