Human beings are known for a myriad of different things, but most importantly, they are known for getting better on a consistent basis. This tendency to improve, no matter the situation, has brought the world …
Human beings are known for a myriad of different things, but most importantly, they are known for getting better on a consistent basis. This tendency to improve, no matter the situation, has brought the world some huge milestones, with technology emerging as quite a major member of the group. The reason why we hold technology in such a high regard is, by and large, predicated upon its skill-set, which ushered us towards a reality that nobody could have ever imagined otherwise. Nevertheless, if we look beyond the surface for a second, it will become clear how the whole runner was also very much inspired from the way we applied those skills across a real world environment. The latter component, in fact, did a lot to give the creation a spectrum-wide presence, and as a result, initiate a full-blown tech revolution. Of course, this revolution eventually went on to scale up the human experience through some outright unique avenues, but even after achieving a feat so notable, technology will somehow continue to bring forth the right goods. The same has turned more and more evident in recent times, and assuming one new discovery ends up with the desired impact, it will only put that trend on a higher pedestal moving forward.
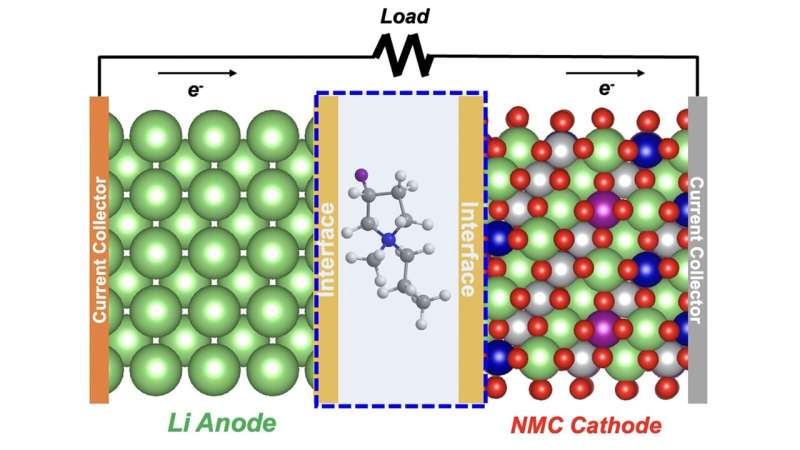
The researching team at the U.S. Department of Energy’s (DOE) Argonne National Laboratory has successfully made a discovery, which states that fluoride electrolyte, an ingredient more commonly found in toothpastes, can be used to protect new-age batteries from a decline in performance. To understand the significance of such a development, we must acknowledge the developments that have been lately transpiring across the automotive industry. With non-lithium-ion batteries becoming a mainstream concept, the cars are now able to store more energy and cover greater distance than ever before. However, despite its enormous potential for the future, the concept faces a major challenge that translates to the promised high energy density declining quite dramatically after repeated charge and discharge. The stated team of researchers, fortunately enough, has found a solution in switching up the electrolyte, a liquid through which lithium ions move between cathode and anode to implement charge and discharge. You see, under current battery structure, this electrolyte element has proven incapable of forming an adequate protective layer on the anode surface during the first few cycles. Named solid-electrolyte-interphase (SEI), the proverbial layer is notably significant, considering how that is what ensures the ion movement remains protected from eventual wear and tear.
In place of the current electrolyte, the researchers have proposed a new fluoride solvent that maintains a robust protective layer for well over hundred cycles. On a more actionable note, the solvent places a positively-charged fluorinated component alongside a negatively-charged fluorinated component to create an ionic liquid consisting both positive and negative ions.
“The key difference in our new electrolyte is the substitution of fluorine for hydrogen atoms in the ring-like structure of the cation part of the ionic liquid,” said Zhengcheng (John) Zhang, a group leader in Argonne’s Chemical Sciences and Engineering division.” This made all the difference in maintaining high performance for hundreds of cycles in a test lithium metal cell.”
To test out their concept, the team conducted some simulations on Argonne Leadership Computing Facility’s Theta supercomputer where it was revealed that the fluorine cations stick to and accumulate on the anode and cathode surfaces before any charge-discharge cycling. Next up, in the early stages of cycling, they observed a resilient SEI layer being formed, a layer which is superior to the outcome we have seen with previous electrolytes.
Apart from overcoming durability barriers, the new electrolyte also offers better financial feasibility due to a lower cost, while simultaneously putting on the table higher purity levels and a more streamlined yield procedure. In case it still doesn’t sound attractive enough of a prospect, then it might be worth acknowledging that the new electrolyte is also a more environmental-friendly alternative as it uses much less solvent, a volatile element which can release contaminants into the environment.
“Lithium metal batteries with our fluorinated cation electrolyte could considerably boost the electric vehicle industry,” Zhang said. “And the usefulness of this electrolyte undoubtedly extends to other types of advanced battery systems beyond lithium ion.”